
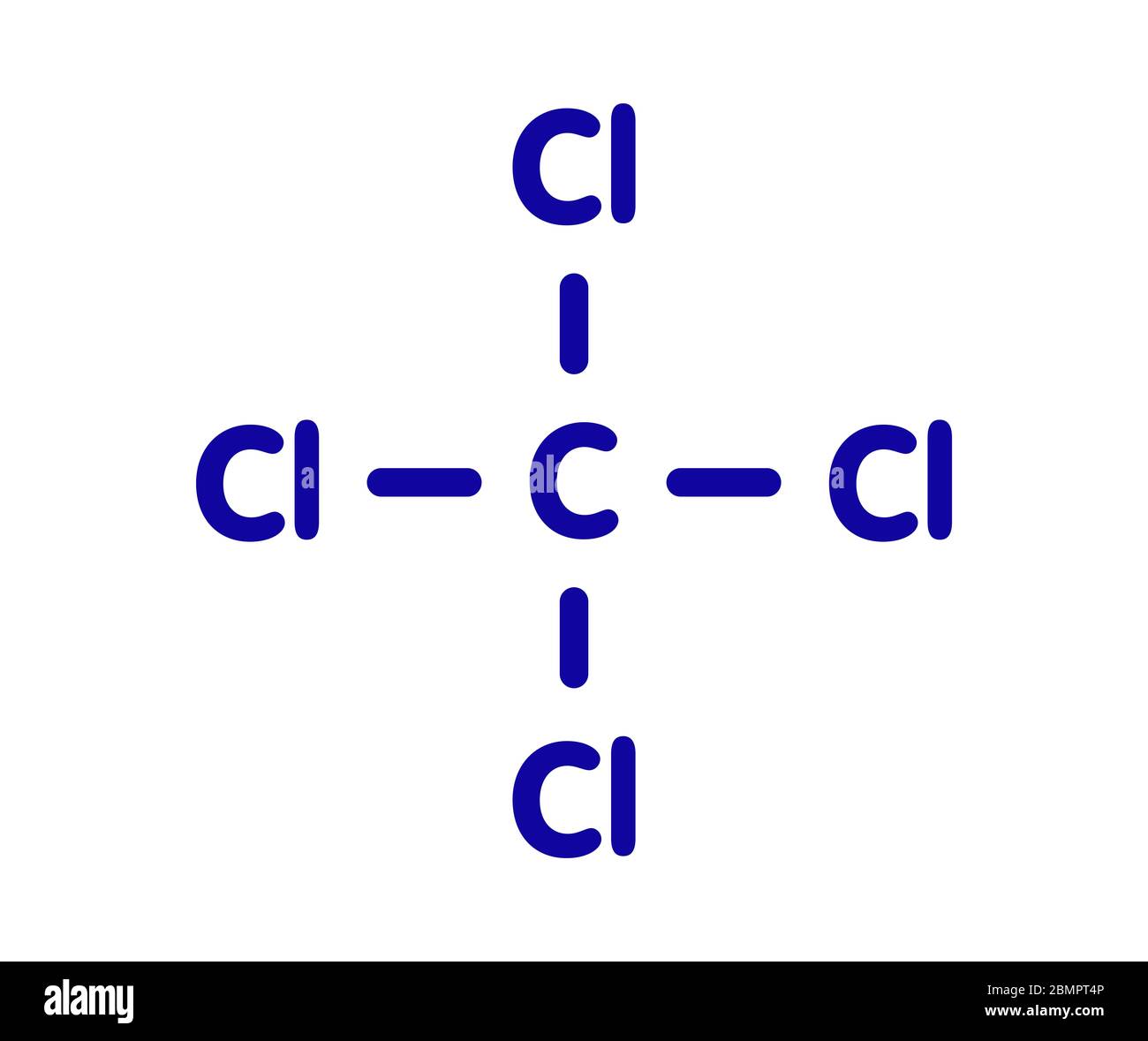
Putting these pieces together gives the name carbon tetrachloride for this compound. Since carbon monoxide is not an ionic compound it technically doesnt have an ionic formula. The elements in (N2O4) are both nonmetals, rather than a metal and a nonmetal. Is carbon tetrafluoride molecular or ionic carbon tetrachloride is a molecular compound. so this compound contains both ionic and covalent bonds. a) Na, F b) Be, O C) Al, S d) K, Se e) Li, N f) Mg, P g) Ca, H Give the correct chemical formula for each of the following binary compounds: nonmetal-nonmetal. The chemical formula of a simple covalent compound can be determined from its name. Carbon tetrachloride, also known by many other names (such as carbon tet for short and tetrachloromethane, also recognised by the IUPAC) is a chemical compound with the chemical formula CCl4. CAS Registry Number: Chemical structure: This structure is also available as a 2d Mol file 3d SD file The 3d structure may be viewed using. Write the chemical formula(s) of the ionic compounds formed between each of the following pairs (metal and nonmetal) of elements. IUPAC Standard InChIKey:VZGDMQKNWNREIO-UHFFFAOYSA-N. Carbon atom contains eight electrons in outer shell and chlorine atom also have eight electrons, so they are completing their octet by the sharing of electrons with each other. This is to show that the subscript applies to the entire polyatomic ion. IUPAC Standard InChI:InChI1S/CCl4/c2-1 (3,4)5. Science Chemistry Write the ionic or molecular formula for the following compounds appropriately. Explanation: In carbon tetrachloride, chlorine and carbon are bounded with each other by single covalent bond.
The strongest forces between HF molecules are. In water, the melting point is unusually high because of. Give the correct formula and chemical name for the ionic compound: Ca2+ and CO32-Give the correct formula and chemical name for the ionic compound: Fe3+ and CO32-. Answer 4: Yes, because it contains polar bond (s) The main interactions between molecules of hydrogen chloride are examples of.

If more than one of a particular polyatomic ion is needed to balance the charge, the entire formula for the polyatomic ion must be enclosed in parentheses, and the numerical subscript is placed outside the parentheses. Which is of these is the correct chemical formula for carbon tetrachloride a.

The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance. So what happens is that each chlorine shares one electron with the carbon and the carbon shares one electron with each of the chlorines (that's 4 total). The carbon has 4 electrons in its outer shell (2s 2 2p 2) and each of the four chlorines has 7 (2s 2 2p 5). Trending Questions What is a sentence for octet? What is the sum of all oxidation numbers in any compound? Can use the word crystal lattice in a sentence? Which of these is the substance being dissolved solvent solute solution and solubility? Is fire a matter or energy? Why does lead taste sweet? All electrons orbit the nucleus of an atom at the same level.\): Some Polyatomic Ions Name An example is carbon tetrachloride (CCl 4 - we'll get to naming in a bit).


 0 kommentar(er)
0 kommentar(er)
